The CEPI-funded study will evaluate the safety, tolerability, and immunogenicity of IAVI’s single dose Lassa fever vaccine candidate
Key takeaways:
- Participants in Nigeria have been vaccinated with a Lassa fever vaccine candidate in the first Phase 2 clinical trial of any Lassa vaccine.
- Lassa fever is a deadly hemorrhagic fever common to West Africa. No approved vaccine currently exists.
- More than 600 participants in Ghana, Liberia, and Nigeria are expected to enroll in the IAVI-sponsored trial, funded by CEPI.
NEW YORK, NY / ACCESSWIRE / April 4, 2024 / Participants at HJF Medical Research International in Abuja, Nigeria, have been vaccinated in the first Phase 2 clinical trial of a Lassa fever virus (LASV) vaccine candidate to date, according to IAVI, a nonprofit scientific research organization and the trial sponsor. The study (IAVI C105/PREVAIL15) is funded by CEPI, an innovative global partnership working to accelerate the development of vaccines against epidemic and pandemic threats.
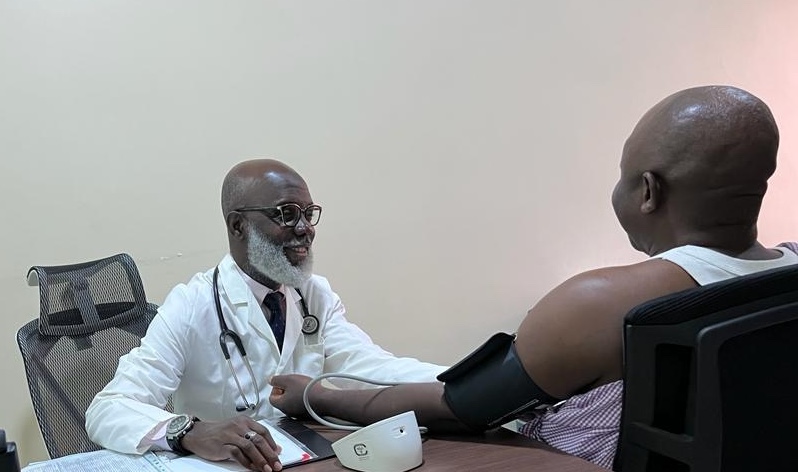
The trial in Nigeria has been designed in consultation with in-country partners, including the Nigeria Centre for Disease Control and Prevention and the Nigeria Lassa Vaccine Taskforce. HJF Medical Research International, Ltd/Gte (HJFMRI) has been conducting infectious diseases research in collaboration with HJF and the Walter Reed Army Institute of Research in Nigeria since 2014. In addition to Nigeria, and pending regulatory approval, IAVI expects to enroll additional participants at the Noguchi Memorial Institute for Medical Research (NMIMR), College of Health Sciences, University of Ghana, and the Partnership for Research on Vaccines and Infectious Diseases in Liberia (PREVAIL)1.
"Nigerian clinicians, scientists and community members are key leaders in this international collaboration, which will ensure that vaccine development incorporates local context, experience and perspectives while fostering sustainable in-country research capacity and partnerships," said Dr. Abdulwasiu Bolaji Tiamiyu, clinical research center director, HJFMRI, and principal investigator of IAVI C105 in Nigeria.
No approved vaccine currently exists for LASV, which causes an acute viral hemorrhagic illness called Lassa fever. Given its potential to cause a public health emergency of international concern, LASV is included in the World Health Organization R&D Blueprint of priority pathogens for which there is an urgent need for accelerated research and development (R&D) and countermeasures2. IAVI and members of the global Viral Hemorrhagic Fever Consortium have been collaborating since 2018 to accelerate clinical development of IAVI’s single-dose LASV vaccine candidate in studies supported and funded by CEPI and the European & Developing Countries Clinical Trials Partnership (EDCTP). Read more.
An estimated 5,000 people die each year from LASV, and about 300,000 people fall ill across West Africa annually – though the true disease burden is thought to be much higher. Nigeria, Liberia, Sierra Leone, and Guinea are most affected, but increasingly, neighboring countries are experiencing their own emerging outbreaks, with travelers occasionally carrying infections to other regions. Although most people who develop LASV have mild or no symptoms, approximately 20% will develop more serious symptoms including widespread bleeding and major organ failure. Approximately a third of those infected with LASV experience associated deafness, sometimes resulting in lifelong disability. Children under 10 years old, pregnant people, and health care workers are especially vulnerable to LASV infection.
"Continued outbreaks of Lassa fever and the emergence of Ebola Sudan in Uganda both underscore the need to have vaccines for known disease threats available for evaluation and use during outbreak situations – the overarching goal of IAVI’s emerging infectious disease program." said Swati Gupta, DrPH, MPH, vice president and head of emerging infectious diseases and epidemiology, IAVI. "IAVI C105 is an important step toward attaining eventual licensure of a Lassa fever vaccine, should IAVI’s vaccine candidate prove to have an acceptable safety profile and be efficacious. We are grateful to our funders, our clinical partners, and our consortium collaborators for their continued support of IAVI and commitment to a more secure world."
"Lassa fever has long been a threat to much of West Africa, and factors like climate change and population growth could exacerbate its transmission in coming decades," explains Dr. Richard Hatchett, CEO of CEPI. "The world urgently needs a Lassa vaccine for routine immunisation. The initiation of IAVI’s new Phase 2 trial – the most advanced Lassa vaccine trial to date – is an important milestone in public health and signals that better tools to manage and prevent outbreaks are coming. We look forward to continuing to collaborate with IAVI and regional partners to advance this promising vaccine as quickly as possible."
IAVI’s LASV vaccine candidate was well-tolerated and immunogenic among participants in both the U.S. and Liberian cohorts of IAVI’s Phase I LASV vaccine trial (IAVI C102). Robust immune responses appear to be sustained for up to one year after vaccination3. In the Phase 2 study, researchers will evaluate the vaccine candidate’s safety, tolerability, and immunogenicity at two different dosage levels in adults, including people living with HIV, as well as in adolescents and in children two years of age and older. Approximately 612 participants will be enrolled and followed for six months after vaccination to monitor their safety and immune responses. A subset of participants will be followed for an additional two years for extended safety and immunogenicity.
CEPI and IAVI are united in their commitment to global equitable access. Should the candidate be found to be safe and efficacious in clinical testing, IAVI is committed to making its Lassa vaccine affordable and accessible to all populations in need.
Harnessing a proven platform technology for global preparedness
IAVI’s LASV vaccine candidate uses the same recombinant vesicular stomatitis virus (rVSV) vector platform as ERVEBO®, Merck’s single-dose Zaire ebolavirus (ZEBOV) vaccine, which is licensed in North America, Europe, and 10 African countries. The rVSV platform has been used extensively in adults and children4.
IAVI’s goal is to develop and test rVSV-based vaccines against emerging infectious disease pathogens that pose epidemic threats as part of an overall preventive strategy which includes routine immunization as appropriate as well as stockpiles which can be rapidly deployed for larger outbreaks as needed. IAVI’s EID portfolio includes a Sudan ebolavirus vaccine candidate supported by BARDA; a Lassa fever vaccine candidate supported by CEPI and EDCTP; a Marburg virus vaccine candidate supported by BARDA and the Defense Threat Reduction Agency (DTRA) of the U.S. Department of Defense (DOD); and an intranasal SARS-CoV-2 vaccine candidate supported by DOD DTRA and the Japan Ministry of Finance.
Much of the R&D on IAVI’s rVSV platform is performed at the IAVI Vaccine Design and Development Lab (DDL) in Brooklyn, New York. The DDL is one of the world’s leading viral vector vaccine R&D labs, known for innovation and generation of novel vaccine design concepts. Scientists with IAVI’s Human Immunology Laboratory (HIL) in London, U.K., are involved in processing participant samples and developing the analytical assays needed to evaluate IAVI C105 participants’ immune responses. They are also facilitating technology transfer of the assays to West African partner institutions.
IAVI’s LASV vaccine candidate was manufactured by Batavia Biosciences in Leiden, The Netherlands, a contract-development and manufacturing organization focused on delivering sustainable, low-cost manufacturing solutions in the field of infectious disease and cancer. Through its partnership with Batavia, IAVI intends to develop an end-to-end platform for flexible, low-cost production of epidemic preparedness vaccines.
Results from IAVI C105 are expected in 2025 and will be made available through open-access publications and via scientific meetings to ensure all can benefit from the research.
###
IAVI Media Contact
Rose Catlos
Rcatlos@iavi.org
212-847-1049
CEPI Media Contact
press@cepi.net
+44 7387 055214
HJFMRI Media Contact
Lisa Reilly
lreilly@Global-ID.org
301-339-3566
About IAVI
IAVI is a nonprofit scientific research organization dedicated to addressing urgent, unmet global health challenges including HIV, tuberculosis, and emerging infectious diseases. Its mission is to translate scientific discoveries into affordable, globally accessible public health solutions. Read more at iavi.org.
Follow IAVI on Facebook, LinkedIn, Instagram, and YouTube, and subscribe to our news updates.
About CEPI
CEPI was launched in 2017 as an innovative partnership between public, private, philanthropic and civil organisations. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need. CEPI has supported the development of more than 50 vaccine candidates or platform technologies against multiple known high-risk pathogens or a future Disease X. Central to CEPI’s pandemic-beating five-year plan for 2022-2026 is the ‘100 Days Mission’ to compress the time taken to develop safe, effective, globally accessible vaccines against new threats to just 100 days.
About IAVI’s rVSV vaccine candidates
IAVI holds a nonexclusive license to the rVSV vaccine candidates from the Public Health Agency of Canada (PHAC). The vector was developed by scientists at PHAC’s National Microbiology Laboratory.
IAVI initially developed its rVSV vector for HIV vaccine candidates and has since expanded its use to the development of vaccines addressing emerging infectious diseases (Lassa Fever, Marburg, Sudan ebolavirus, and COVID-19).
Funders who have made the development of IAVI’s rVSV-vectored vaccine candidates possible include the Bill & Melinda Gates Foundation; the Government of Canada; the Danish Ministry of Foreign Affairs; the Government of Japan; the Irish Department of Foreign Affairs and Trade; the Netherlands Ministry of Foreign Affairs; the Norwegian Agency for Development Cooperation; the U.K. Foreign, Commonwealth & Development Office; the U.S. National Institutes of Health; and through the generous support of the American people from the United States Agency for International Development.
This project is funded in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority under contract number 75A50121C00077.
1 PREVAIL is a Liberia-U.S. clinical research collaboration established in 2014 by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the Ministry of Health in Liberia. In 2015, PREVAIL participated in a clinical trial (PREVAIL 1) of Merck’s now-licensed Ebola Zaire vaccine ERVEBO®, which uses the same recombinant vesicular stomatitis virus (rVSV) vector backbone as the candidates in IAVI’s emerging infectious diseases (EIDs) vaccine development portfolio, including the IAVI LASV vaccine candidate being evaluated in a Phase I clinical trial.
SOURCE: IAVI
View the original press release on accesswire.com
